We have a broad portfolio of programs
in clinical and research stages
Lazertinib
-
01.About the Drug
Lazertinib is an innovative, synthetic, low-molecular-weight drug that treats non-small cell lung cancer (NSCLC) by selectively inhibiting activated mutant forms of epidermal growth factor receptor (EGFR).
EGFR belongs to the receptor tyrosine kinase family and is widely used as a target for the treatment of lung cancer.
However, although the 1st and 2nd generation inhibitors show high therapeutic efficacy at the beginning of treatment, most patients were reported to have experienced relapse within 1 to 2 years, leading to their deaths.
This is known to be caused by the occurrence of new mutations in the EGFR within the cancer tissues, which leads to the development of resistance to existing targeted therapeutics. Since then, Osimertinib (Targrisso®) has been developed as a 3rd generation inhibitor and widely used as a first-line and second-line treatment.-
1st generation treatment
Erlotinib (Tarceva®)
Gefitinib (Iressa®) -
2nd generation treatment
Afatinib (Gilotrif®)
-
3rd generation treatment
Osimertinib (Targrisso®)
Lazertinib (Lazcluze™)
In spite of its low inhibitory effect against normal EGFR, Lazertinib exhibits potent irreversible inhibition against EGFRs with single mutations (Del19, L858R, Exon20ins, etc.) and double mutations (Del19/T790M or L858R/T790M).
Thanks to its excellent blood brain barrier (BBB) permeability, Lazertinib also displays excellent anticancer activity even in patients with brain metastasis.
In addition to its excellent efficacy as described above, Lazertinib shows higher tolerance and lower skin toxicity-related side effects than existing drugs, so it is expected to become an innovative lung cancer treatment that is superior to the existing 3rd generation treatments.Lazertinib was licensed out from Genosco/Oscotec to Yuhan Corporation in July 2015, then in November 2018 it was licensed out again from Yuhan Corporation to Janssen, a subsidiary of Johnson & Johnson. In January 2021 and June 2023, Lazertinib was approved by the Korean Ministry of Food and Drug Safety for the treatment of non-small cell lung cancer (NSCLC) as a second-line and first-line therapy, respectively (brand name: LECLAZA®). Additionally, in August 2024, the combination therapy of Lazertinib and Amivantamab was approved by the U.S. FDA as a first-line treatment for NSCLC. In December 2024, the combination therapy was approved in Europe, followed by approval in Japan in March 2025 as a first-line treatment for NSCLC.
-
-
02.Current Progress
Lazertinib is undergoing phase 3 clinical trials as mono-therapy and combo-therapy with
Janssen’s Amivantamab (EGFR/C-met Antibody) for treatment of NSCLC. -
03.About the Disease
Lung cancer refers to a malignant tumor in the lungs. Currently, it is one of the top five cancers with high incidence in Korea and is notorious as a cancer with the highest fatality rate among them.
Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers and activated mutant forms of epidermal growth factor receptor (EGFR), in particular, are closely associated with poor prognosis.
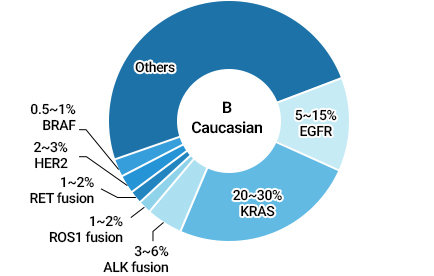
40-55% of Asians and 5-15% of Caucasians have these mutations in their cancerous tissues.Subtypes of Lung Cancer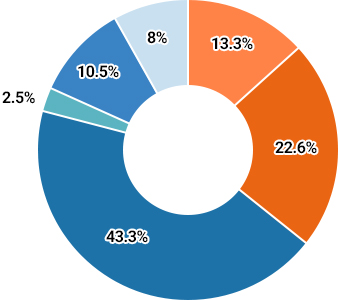
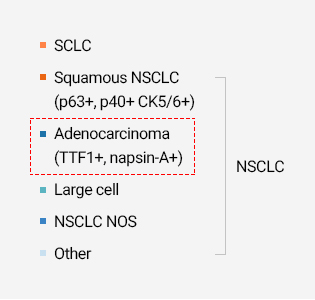
Source : Naidoo J, Drilon A. Molecular diagnostic testing in non-small cell lung cancer. Am J Hematol Oncol. 2014;10(4):4–11
Various Driver Mutations in NSCLC Patients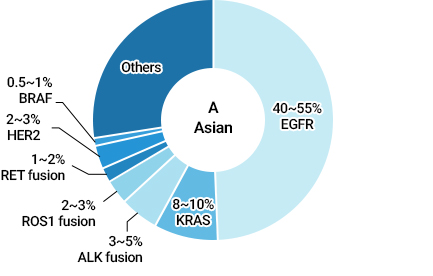
 Different Mutations among EGFR Mutations in NSCLC Patients
Different Mutations among EGFR Mutations in NSCLC Patients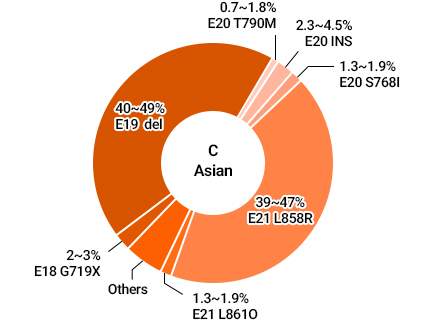
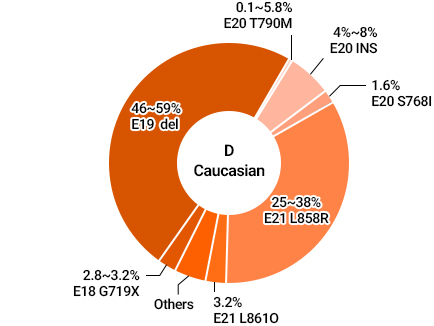
Source : Li et al, Determining TKI Sensitivity of EGFR Uncommon Mutations in NSCLC, Oncol Rep. 2017 Mar;37(3):1347-1358
Many patients treated with the 1st and the 2nd generation drugs, which are currently used as treatments for NSCLC, are likely to develop resistance within 1 to 2 years after the start of treatment. Moreover, even when the 3rd generation drugs are administered as a first-line or second-line treatment, various kinds of additional resistance are developed, which continuously generates unmet demand.